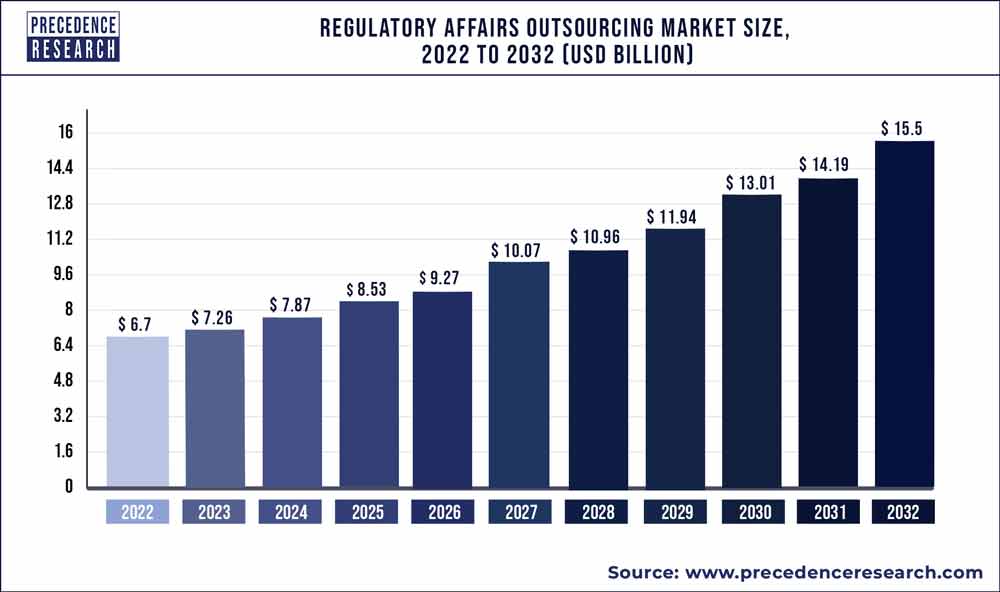
Precedence Research analyzes the new publication titled “Regulatory Affairs Outsourcing Market (By Service: Legal Representation, Regulatory Consulting, Product Registration & Clinical Trial Application, Regulatory Writing & Publication, Others; By Category: Biologics, Drugs, Medical Devices; By End User: Medical Device Company, Biotechnology Company, and Pharmaceutical Company; By Indication: Neurology, Oncology, Immunology, Cardiology, Others) - Global Industry Analysis, Size, Share, Growth, Trends, Regional Outlook, and Forecast 2021 – 2030”, As per the report, the regulatory affairs outsourcing market size was US$ 6.5 billion in 2021. It is projected to grow US$ 16.6 billion by 2030 at a CAGR of 10.2% in the forecast period.
Download the FREE Sample Report (Including TOC, List of Tables & Figures, and Chart) @ https://www.precedenceresearch.com/sample/1373
Market Overview
A recent study by Precedence Research on the regulatory affairs outsourcing market offers a forecast for 2021 and 2030. The study analyzes crucial trends that are currently determining the growth of the regulatory affairs outsourcing market. This report explicates on vital dynamics such as the drivers, restraints, and opportunities for key market players, along with key stakeholders as well as emerging players associated with the manufacturing of regulatory affairs outsourcing. The study also provides the dynamics that are responsible for influencing the future status of this market over the forecast period.
A detailed assessment of the regulatory affairs outsourcing market value chain analysis, business execution, and supply chain analysis across regional markets has been covered in the report. A list of prominent companies operating in the regulatory affairs outsourcing market along with their product portfolio enhances the reliability of this comprehensive research study.
Highlights of the Report:
- Market Penetration: Comprehensive information on the product portfolios of the top players in the market.
- Product Development/Innovation: Detailed insights on the upcoming technologies, R&D activities, and product launches in the market
- Competitive Assessment: In-depth assessment of the market strategies, geographic and business segments of the leading players in the market
- Market Development: Comprehensive information about emerging markets. This report analyzes the market for various segments across geographies
- Market Diversification: Exhaustive information about new products, untapped geographies, recent developments, and investments in the market
Key Points
- Primary Research
- Secondary Research
- Market Size Estimation
- Market Dynamics
- Drivers
- Restraints
- Opportunities
- Challenges
- Macroeconomic Indicators
- Technology Trends and Assessment
- Market Factor Analysis
- Porter’s Five Forces Analysis
- Bargaining Power Of Suppliers
- Bargaining Power Of Buyers
- Threat Of New Entrants
- Threat Of Substitutes
- Intensity Of Rivalry
- Value Chain Analysis
- Investment Feasibility Analysis
- Pricing Analysis
Research Methodology
Secondary Research
It involves company databases such as Hoover's: This assists us recognize financial information, the structure of the market participants and industry's competitive landscape.
The secondary research sources referred in the process are as follows:
- Governmental bodies, and organizations creating economic policies
- National and international social welfare institutions
- Company websites, financial reports and SEC filings, broker and investor reports
- Related patent and regulatory databases
- Statistical databases and market reports
- Corporate Presentations, news, press release, and specification sheet of Manufacturers
Primary Research
Primary research includes face-to-face interviews, online surveys, and telephonic interviews.
- Means of primary research: Email interactions, telephonic discussions and Questionnaire-based research etc.
- In order to validate our research findings and analysis we conduct primary interviews of key industry participants. Insights from primary respondents help in validating the secondary research findings. It also develops Research Team’s expertise and market understanding.
Industry participants involved in this research study include:
- CEOs, VPs, market intelligence managers
- Procuring and national sales managers technical personnel, distributors and resellers
- Research analysts and key opinion leaders from various domains
Competition Landscape
In the final section of the report on the global regulatory affairs outsourcing market, a 'dashboard view' of the companies has been provided to compare the current market scenario and the contribution of companies to the global regulatory affairs outsourcing market. Moreover, the report is primarily designed to provide clients with an objective and detailed comparative assessment of key providers specific to segments of the global market. Report audiences can gain segment-specific manufacturer insights to identify and evaluate key competitors based on an in-depth assessment of their capabilities and success in the regulatory affairs outsourcing marketplace.
Some of the prominent players in the global regulatory affairs outsourcing market include:
- Medpace
- ICON Plc
- WuXiAppTec, Inc.
- Covance
- Genpact Ltd.
- Pharmaceutical Product Development LLC.
- Freyr
- PRA Health Sciences
- Criterium, Inc.
- Accell Clinical Research, LLC.
Market Segmentation
Segments of the global regulatory affairs outsourcing market have been analyzed in terms of their market share to understand an individual segment’s relative contribution to market growth. This detailed level of information is important for identifying key trends in the global regulatory affairs outsourcing market.
Market Segments as below:
By Service
- Legal Representation
- Regulatory Consulting
- Product Registration & Clinical Trial Application
- Regulatory Writing & Publication
- Others
By Category
- Biologics
- Drugs
- Medical Devices
By End User
- Medical Device Company
- Biotechnology Company
- Pharmaceutical Company
By Indication
- Neurology
- Oncology
- Immunology
- Cardiology
- Others
By Stage
- Clinical
- Preclinical
- Post Market Authorization
By Geography
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Asia Pacific
- China
- India
- Japan
- South Korea
- Rest of the World
Table of Content
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Regulatory Affairs Outsourcing Market
5.1. COVID-19 Landscape: Regulatory Affairs Outsourcing Industry Impact
5.2. COVID 19 - Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Regulatory Affairs Outsourcing Market, By Service
8.1. Regulatory Affairs Outsourcing Market, by Service, 2021-2030
8.1.1. Legal Representation
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Regulatory Consulting
8.1.2.1. Market Revenue and Forecast (2017-2030)
8.1.3. Product Registration & Clinical Trial Application
8.1.3.1. Market Revenue and Forecast (2017-2030)
8.1.4. Regulatory Writing & Publication
8.1.4.1. Market Revenue and Forecast (2017-2030)
8.1.5. Others
8.1.5.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Regulatory Affairs Outsourcing Market, By Category
9.1. Regulatory Affairs Outsourcing Market, by Category, 2021-2030
9.1.1. Biologics
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Drugs
9.1.2.1. Market Revenue and Forecast (2017-2030)
9.1.3. Medical Devices
9.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 10. Global Regulatory Affairs Outsourcing Market, By End User
10.1. Regulatory Affairs Outsourcing Market, by End User, 2021-2030
10.1.1. Medical Device Company
10.1.1.1. Market Revenue and Forecast (2017-2030)
10.1.2. Biotechnology Company
10.1.2.1. Market Revenue and Forecast (2017-2030)
10.1.3. Pharmaceutical Company
10.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 11. Global Regulatory Affairs Outsourcing Market, By Indication
11.1. Regulatory Affairs Outsourcing Market, by Indication, 2021-2030
11.1.1. Neurology
11.1.1.1. Market Revenue and Forecast (2017-2030)
11.1.2. Oncology
11.1.2.1. Market Revenue and Forecast (2017-2030)
11.1.3. Immunology
11.1.3.1. Market Revenue and Forecast (2017-2030)
11.1.4. Cardiology
11.1.4.1. Market Revenue and Forecast (2017-2030)
11.1.5. Others
11.1.5.1. Market Revenue and Forecast (2017-2030)
Chapter 12. Global Regulatory Affairs Outsourcing Market, By Stage
12.1. Regulatory Affairs Outsourcing Market, by Stage, 2021-2030
12.1.1. Clinical
12.1.1.1. Market Revenue and Forecast (2017-2030)
12.1.2. Preclinical
12.1.2.1. Market Revenue and Forecast (2017-2030)
12.1.3. Post Market Authorization
12.1.3.1.Market Revenue and Forecast (2017-2030)
Chapter 13. Global Regulatory Affairs Outsourcing Market, Regional Estimates and Trend Forecast
13.1. North America
13.1.1. Market Revenue and Forecast, by Service (2017-2030)
13.1.2. Market Revenue and Forecast, by Category (2017-2030)
13.1.3. Market Revenue and Forecast, by End User (2017-2030)
13.1.4. Market Revenue and Forecast, by Indication (2017-2030)
13.1.5. Market Revenue and Forecast, by Stage (2017-2030)
13.1.6. U.S.
13.1.6.1. Market Revenue and Forecast, by Service (2017-2030)
13.1.6.2. Market Revenue and Forecast, by Category (2017-2030)
13.1.6.3. Market Revenue and Forecast, by End User (2017-2030)
13.1.6.4. Market Revenue and Forecast, by Indication (2017-2030)
13.1.7. Market Revenue and Forecast, by Stage (2017-2030)
13.1.8. Rest of North America
13.1.8.1. Market Revenue and Forecast, by Service (2017-2030)
13.1.8.2. Market Revenue and Forecast, by Category (2017-2030)
13.1.8.3. Market Revenue and Forecast, by End User (2017-2030)
13.1.8.4. Market Revenue and Forecast, by Indication (2017-2030)
13.1.8.5. Market Revenue and Forecast, by Stage (2017-2030)
13.2. Europe
13.2.1. Market Revenue and Forecast, by Service (2017-2030)
13.2.2. Market Revenue and Forecast, by Category (2017-2030)
13.2.3. Market Revenue and Forecast, by End User (2017-2030)
13.2.4. Market Revenue and Forecast, by Indication (2017-2030)
13.2.5. Market Revenue and Forecast, by Stage (2017-2030)
13.2.6. UK
13.2.6.1. Market Revenue and Forecast, by Service (2017-2030)
13.2.6.2. Market Revenue and Forecast, by Category (2017-2030)
13.2.6.3. Market Revenue and Forecast, by End User (2017-2030)
13.2.7. Market Revenue and Forecast, by Indication (2017-2030)
13.2.8. Market Revenue and Forecast, by Stage (2017-2030)
13.2.9. Germany
13.2.9.1. Market Revenue and Forecast, by Service (2017-2030)
13.2.9.2. Market Revenue and Forecast, by Category (2017-2030)
13.2.9.3. Market Revenue and Forecast, by End User (2017-2030)
13.2.10. Market Revenue and Forecast, by Indication (2017-2030)
13.2.11. Market Revenue and Forecast, by Stage (2017-2030)
13.2.12. France
13.2.12.1. Market Revenue and Forecast, by Service (2017-2030)
13.2.12.2. Market Revenue and Forecast, by Category (2017-2030)
13.2.12.3. Market Revenue and Forecast, by End User (2017-2030)
13.2.12.4. Market Revenue and Forecast, by Indication (2017-2030)
13.2.13. Market Revenue and Forecast, by Stage (2017-2030)
13.2.14. Rest of Europe
13.2.14.1. Market Revenue and Forecast, by Service (2017-2030)
13.2.14.2. Market Revenue and Forecast, by Category (2017-2030)
13.2.14.3. Market Revenue and Forecast, by End User (2017-2030)
13.2.14.4. Market Revenue and Forecast, by Indication (2017-2030)
13.2.15. Market Revenue and Forecast, by Stage (2017-2030)
13.3. APAC
13.3.1. Market Revenue and Forecast, by Service (2017-2030)
13.3.2. Market Revenue and Forecast, by Category (2017-2030)
13.3.3. Market Revenue and Forecast, by End User (2017-2030)
13.3.4. Market Revenue and Forecast, by Indication (2017-2030)
13.3.5. Market Revenue and Forecast, by Stage (2017-2030)
13.3.6. India
13.3.6.1. Market Revenue and Forecast, by Service (2017-2030)
13.3.6.2. Market Revenue and Forecast, by Category (2017-2030)
13.3.6.3. Market Revenue and Forecast, by End User (2017-2030)
13.3.6.4. Market Revenue and Forecast, by Indication (2017-2030)
13.3.7. Market Revenue and Forecast, by Stage (2017-2030)
13.3.8. China
13.3.8.1. Market Revenue and Forecast, by Service (2017-2030)
13.3.8.2. Market Revenue and Forecast, by Category (2017-2030)
13.3.8.3. Market Revenue and Forecast, by End User (2017-2030)
13.3.8.4. Market Revenue and Forecast, by Indication (2017-2030)
13.3.9. Market Revenue and Forecast, by Stage (2017-2030)
13.3.10. Japan
13.3.10.1. Market Revenue and Forecast, by Service (2017-2030)
13.3.10.2. Market Revenue and Forecast, by Category (2017-2030)
13.3.10.3. Market Revenue and Forecast, by End User (2017-2030)
13.3.10.4. Market Revenue and Forecast, by Indication (2017-2030)
13.3.10.5. Market Revenue and Forecast, by Stage (2017-2030)
13.3.11. Rest of APAC
13.3.11.1. Market Revenue and Forecast, by Service (2017-2030)
13.3.11.2. Market Revenue and Forecast, by Category (2017-2030)
13.3.11.3. Market Revenue and Forecast, by End User (2017-2030)
13.3.11.4. Market Revenue and Forecast, by Indication (2017-2030)
13.3.11.5. Market Revenue and Forecast, by Stage (2017-2030)
13.4. MEA
13.4.1. Market Revenue and Forecast, by Service (2017-2030)
13.4.2. Market Revenue and Forecast, by Category (2017-2030)
13.4.3. Market Revenue and Forecast, by End User (2017-2030)
13.4.4. Market Revenue and Forecast, by Indication (2017-2030)
13.4.5. Market Revenue and Forecast, by Stage (2017-2030)
13.4.6. GCC
13.4.6.1. Market Revenue and Forecast, by Service (2017-2030)
13.4.6.2. Market Revenue and Forecast, by Category (2017-2030)
13.4.6.3. Market Revenue and Forecast, by End User (2017-2030)
13.4.6.4. Market Revenue and Forecast, by Indication (2017-2030)
13.4.7. Market Revenue and Forecast, by Stage (2017-2030)
13.4.8. North Africa
13.4.8.1. Market Revenue and Forecast, by Service (2017-2030)
13.4.8.2. Market Revenue and Forecast, by Category (2017-2030)
13.4.8.3. Market Revenue and Forecast, by End User (2017-2030)
13.4.8.4. Market Revenue and Forecast, by Indication (2017-2030)
13.4.9. Market Revenue and Forecast, by Stage (2017-2030)
13.4.10. South Africa
13.4.10.1. Market Revenue and Forecast, by Service (2017-2030)
13.4.10.2. Market Revenue and Forecast, by Category (2017-2030)
13.4.10.3. Market Revenue and Forecast, by End User (2017-2030)
13.4.10.4. Market Revenue and Forecast, by Indication (2017-2030)
13.4.10.5. Market Revenue and Forecast, by Stage (2017-2030)
13.4.11. Rest of MEA
13.4.11.1. Market Revenue and Forecast, by Service (2017-2030)
13.4.11.2. Market Revenue and Forecast, by Category (2017-2030)
13.4.11.3. Market Revenue and Forecast, by End User (2017-2030)
13.4.11.4. Market Revenue and Forecast, by Indication (2017-2030)
13.4.11.5. Market Revenue and Forecast, by Stage (2017-2030)
13.5. Latin America
13.5.1. Market Revenue and Forecast, by Service (2017-2030)
13.5.2. Market Revenue and Forecast, by Category (2017-2030)
13.5.3. Market Revenue and Forecast, by End User (2017-2030)
13.5.4. Market Revenue and Forecast, by Indication (2017-2030)
13.5.5. Market Revenue and Forecast, by Stage (2017-2030)
13.5.6. Brazil
13.5.6.1. Market Revenue and Forecast, by Service (2017-2030)
13.5.6.2. Market Revenue and Forecast, by Category (2017-2030)
13.5.6.3. Market Revenue and Forecast, by End User (2017-2030)
13.5.6.4. Market Revenue and Forecast, by Indication (2017-2030)
13.5.7. Market Revenue and Forecast, by Stage (2017-2030)
13.5.8. Rest of LATAM
13.5.8.1. Market Revenue and Forecast, by Service (2017-2030)
13.5.8.2. Market Revenue and Forecast, by Category (2017-2030)
13.5.8.3. Market Revenue and Forecast, by End User (2017-2030)
13.5.8.4. Market Revenue and Forecast, by Indication (2017-2030)
13.5.8.5. Market Revenue and Forecast, by Stage (2017-2030)
Chapter 14. Company Profiles
14.1. Medpace
14.1.1. Company Overview
14.1.2. Product Offerings
14.1.3. Financial Performance
14.1.4. Recent Initiatives
14.2. ICON Plc
14.2.1. Company Overview
14.2.2. Product Offerings
14.2.3. Financial Performance
14.2.4. Recent Initiatives
14.3. WuXiAppTec, Inc.
14.3.1. Company Overview
14.3.2. Product Offerings
14.3.3. Financial Performance
14.3.4. Recent Initiatives
14.4. Covance
14.4.1. Company Overview
14.4.2. Product Offerings
14.4.3. Financial Performance
14.4.4. Recent Initiatives
14.5. Genpact Ltd.
14.5.1. Company Overview
14.5.2. Product Offerings
14.5.3. Financial Performance
14.5.4. Recent Initiatives
14.6. Pharmaceutical Product Development LLC.
14.6.1. Company Overview
14.6.2. Product Offerings
14.6.3. Financial Performance
14.6.4. Recent Initiatives
14.7. Freyr
14.7.1. Company Overview
14.7.2. Product Offerings
14.7.3. Financial Performance
14.7.4. Recent Initiatives
14.8. PRA Health Sciences
14.8.1. Company Overview
14.8.2. Product Offerings
14.8.3. Financial Performance
14.8.4. Recent Initiatives
14.9. Criterium, Inc.
14.9.1. Company Overview
14.9.2. Product Offerings
14.9.3. Financial Performance
14.9.4. Recent Initiatives
14.10. Accell Clinical Research, LLC.
14.10.1. Company Overview
14.10.2. Product Offerings
14.10.3. Financial Performance
14.10.4. Recent Initiatives
Chapter 15. Research Methodology
15.1. Primary Research
15.2. Secondary Research
15.3. Assumptions
Chapter 16. Appendix
16.1. About Us
16.2. Glossary of Terms
Thanks for reading you can also get individual chapter-wise sections or region-wise report versions such as North America, Europe, or the Asia Pacific.
Why Buy this Report?
The purpose of Precedence Research's regulatory affairs outsourcing market study is to provide stakeholders with a detailed picture of potential barriers and untapped opportunities. The report contains exclusive information to assist businesses in making informed decisions about how to maintain growth throughout the assessment period.
Buy Full Research Report (Single User License US$ 4500) @ https://www.precedenceresearch.com/checkout/1373
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com



0 Comments