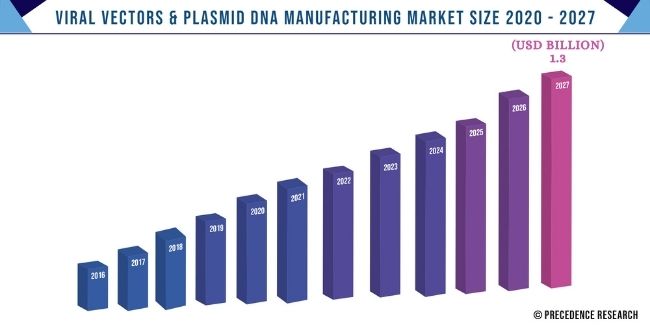
The global viral vectors & plasmid DNA manufacturing market size is expected to reach US$ 1.3 billion by 2027 from US$ 319.01 billion in 2019 and is expected to grow at an impressive double-digit rate of 14.08% from 2020 to 2027.
The study includes drivers and restraints of this market. The study provides an analysis of the global viral vectors & plasmid DNA manufacturing Market for the period 2016-2027, wherein 2020 to 2027 is the forecast period and 2020 is considered as the base year.
Growth Factors
Viral vectors have become ideal choice for gene transferdue to their efficient gene delivery, high transfection efficiency and stable gene expression. Further, upsurge in registration of clinical trials on viral vector-mediated gene therapy is stimulating inclination for viral vectors in gene transfer. Growing pervasiveness of target disorders and diseases, the accessibility of funding for gene therapy development, current research into viral vector-based cell and gene therapies and efficacy of viral vectors in gene therapy delivery are together supporting the market growth. Unexploitedlatent in emergent markets is projected to provideworthwhile growth opportunities for participants in this market. Furthermore, groundbreaking development in the vaccinology is fuelling the demand of these vectors to greater extent. Great amount of clinical and preclinical studies assessing the prospective of vectors in these cutting-edge therapies haveexhibitedfavorable results. As a result of this,several investors are attracted towards this area which is making plasmid and viral vector manufacturing market avigoroussector of investment. Further, it is inspiringfortunate funding activities from both the public and private sectors.
Report is Ready, Immediate Delivery after Payment | Get the Sample Pages of Report@ https://www.precedenceresearch.com/sample/1012
Why should you invest in this report?
If you are aiming to enter the global viral vectors & plasmid DNA manufacturing market, this report is a comprehensive guide that provides crystal clear insights into this niche market. All the major application areas for viral vectors & plasmid DNA manufacturing are covered in this report and information is given on the important regions of the world where this market is likely to boom during the forecast period of 2020-2027, so that you can plan your strategies to enter this market accordingly.
Besides, through this report, you can have a complete grasp of the level of competition you will be facing in this hugely competitive market and if you are an established player in this market already, this report will help you gauge the strategies that your competitors have adopted to stay as market leaders in this market. For new entrants to this market, the voluminous data provided in this report is invaluable.
Report Highlights
- By Vector Type, in 2019, AAV dominated the market with around 22.83% share in terms of revenue of the total market. Lentivirus is projected to grow at highest CAGR of 23.27% during forecast period and anticipated to attain a market share of 17.41% in 2027.
- The global market for the Plasmid DNA was valued at USD 83.5 million in 2019 and is expected to reach USD 407.4 million by 2027.
- By Disease Type, in 2019, cancer dominated the market with around 55.32% share in terms of revenue of the total market. This growth is credited to the rising cancer incidence across the globe. Cancer is also projected to grow at highest CAGR of 22.32% during forecast period.
- By Application Type, in 2019, gene therapy dominated the market with around 37.23% share in terms of revenue of the total market. The global market for the Antisense &RNAi was valued at USD 111.5 million in 2019 and is expected to reach USD 543.4 million by 2027.
- By End-User, in 2019, Biopharmaceutical & Pharmaceutical Companies dominated the market with around 67.45% share in terms of revenue of the total market. Biopharmaceutical & Pharmaceutical Companies is projected to grow at highest CAGR of 22.27% during forecast period.
- Europe Viral Vectors & Plasmid DNA Manufacturing market was estimated at USD 92.7 million in 2019 and is projected to reach value USD 435.2 million by 2027.
- Asia Pacific Viral Vectors & Plasmid DNA Manufacturing market was estimated at USD 56.2 million in 2019 and is projected to reach value USD 294.5 million by 2027.
Drivers
- Rising prevalence of cancer and rare genetic diseases
- Growing research & development of gene therapies in cancer treatment
Market Restraints
- Challenges in executing gene therapies
- High cost associated with gene therapies
Opportunities
- Investment and collaboration in cell and gene therapy sector
- Increase in demand for synthetic genes
Gene therapy is an experimental treatment that comprises incorporating genetic material inside a person’s cells to stopor fight a particular disease. Researchers are studying gene therapy for several diseases including hemophilia, cancer, Parkinson's disease, severe combined immuno-deficiencies, HIV, via numerous dissimilar methods. A gene can be carried to a cell with the help of a carrier known as a vector. Viruses are the most generalkinds of vectors used in gene therapy. Since last few years, several non-viral and viral vectors have been standardized and enhanced.
At present, the much wide spread viral vectors utilized for gene therapies are those based on lentivirus, retrovirus, adenovirus, and AAV vectors. These correspondingly form 8%, 16%, 20%, and 8% of the clinical trialsof active gene therapy. Likewise, plasmid DNA has appeared as the most frequently preferred vectors amid non-viral gene delivery tools. Further, it finds application in production and development of DNA vaccines and viral vectors. There is growing demand for practical manufacturing solutions for viral vectors that can be freely scaled and boosted. This is because gene and cell therapies possess latent to quickly advance via clinical trials to commercialization.With the help of ongoing efforts, several organizations are respectivelyexecutingand developing state-of-the-art solutions intended to scale-up for viral vector manufacturing and speed up process development.
Global Market Revenue (USD Million) and Growth Rate Comparison by Workflow (2016-2027)
| Workflow | 2016 | 2019 | 2027 | CAGR % (2020-2027) | |
| Upstream Processing | 94.60 | 165.45 | 770.72 | 21.27 | % |
| Downstream Processing | 145.60 | 261.13 | 1,301.65 | 22.30 | % |
| Total | 240.20 | 426.58 | 2,072.36 | 21.91 | % |
Key Players & Strategies
Several established organizations have been involved in the production of vectors since the inception of this domain. However, the growing demand for these programs has spurred the establishment of many start-ups as well. Examples include (indicative list, in alphabetical order) Batavia Biosciences, Brammer Bio, GenIBET Biopharmaceuticals, Immune Technology, Lentigen Technology, Luminous Biosciences, Oxford Genetics, SignaGen Laboratories, Vectalys and Virovek. It is also worth highlighting that over 50 academic institutes / non-profit organizations are currently involved in the production of vectors for use in gene therapies.
The demand for clinical grade and research vectors is much than commercial grade vectors and almost many gene therapy entrants are in development stage. Nevertheless, some companieshave are rigorouslymanufacturing commercial scale capacity for vector production. Some of these players includeBioReliance, Aldevron, Eurogentec, Cobra Biologics, Lonza, MassBiologics, FUJIFILM Diosynth Biotechnologies and WuXiAppTec amongst others.
Market Segmentation
By Vector Type
- Adenovirus
- Plasmid DNA
- Lentivirus
- Retrovirus
- AAV
- Others
By Application
- Gene Therapy
- Antisense &RNAi
- Cell Therapy
- Vaccinology
By Workflow
- Upstream Processing
- Vector Recovery/Harvesting
- Vector Amplification & Expansion
- Downstream Processing
- Fill-finish
- Purification
By End-User
- Biopharmaceutical and Pharmaceutical Companies
- Research Institutes
By Disease
- Genetic Disorders
- Cancer
- Infectious Diseases
- Others
Regional Analysis:
The geographical analysis of the global viral vectors & plasmid DNA manufacturing market has been done for North America, Europe, Asia-Pacific, and the Rest of the World.
The North American market is again segmented into the US, Canada, and Mexico. Coming to the European market, it can be segmented further into the UK, Germany, France, Italy, Spain, and the rest. Coming to the Asia-Pacific, the global viral vectors & plasmid DNA manufacturing Market is segmented into China, India, Japan, and Rest of Asia Pacific. Among others, the market is segmented into the Middle East and Africa, (GCC, North Africa, South Africa and Rest of the Middle East & Africa).
Key Questions Answered by the Report:
- What will be the size of the global viral vectors & plasmid DNA manufacturing market in 2027?
- What is the expected CAGR for the viral vectors & plasmid DNA manufacturing market between 2020 and 2027?
- Which are the top players active in this global market?
- What are the key drivers of this global market?
- How will the market situation change in the coming years?
- Which region held the highest market share in this global market?
- What are the common business tactics adopted by players?
- What is the growth outlook of the global viral vectors & plasmid DNA manufacturing market?
TABLE OF CONTENT
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. Market Dynamics Analysis and Trends
5.1. Market Dynamics
5.1.1. Market Drivers
5.1.2. Market Restraints
5.1.3. Market Opportunities
5.2. Porter’s Five Forces Analysis
5.2.1. Bargaining power of suppliers
5.2.2. Bargaining power of buyers
5.2.3. Threat of substitute
5.2.4. Threat of new entrants
5.2.5. Degree of competition
Chapter 6. Competitive Landscape
6.1.1. Company Market Share/Positioning Analysis
6.1.2. Key Strategies Adopted by Players
6.1.3. Vendor Landscape
6.1.3.1. List of Suppliers
6.1.3.2. List of Buyers
Chapter 7. Global Viral Vectors & Plasmid DNA Manufacturing Market, By Vector Type
7.1. Viral Vectors & Plasmid DNA Manufacturing Market, by Vector Type, 2020-2027
7.1.1. Adenovirus
7.1.1.1. Market Revenue and Forecast (2016-2027)
7.1.2. Plasmid DNA
7.1.2.1. Market Revenue and Forecast (2016-2027)
7.1.3. Lentivirus
7.1.3.1. Market Revenue and Forecast (2016-2027)
7.1.4. Retrovirus
7.1.4.1. Market Revenue and Forecast (2016-2027)
7.1.5. AAV
7.1.5.1. Market Revenue and Forecast (2016-2027)
7.1.6. Others
7.1.6.1. Market Revenue and Forecast (2016-2027)
Chapter 8. Global Viral Vectors & Plasmid DNA Manufacturing Market, By Application Type
8.1. Viral Vectors & Plasmid DNA Manufacturing Market, by Application Type, 2020-2027
8.1.1. Gene Therapy
8.1.1.1. Market Revenue and Forecast (2016-2027)
8.1.2. Antisense &RNAi
8.1.2.1. Market Revenue and Forecast (2016-2027)
8.1.3. Cell Therapy
8.1.3.1. Market Revenue and Forecast (2016-2027)
8.1.4. Vaccinology
8.1.4.1. Market Revenue and Forecast (2016-2027)
Chapter 9. Global Viral Vectors & Plasmid DNA Manufacturing Market, By Workflow Type
9.1. Viral Vectors & Plasmid DNA Manufacturing Market, by Workflow Type, 2020-2027
9.1.1. Upstream Processing (Vector Recovery/Harvesting, Vector Amplification & Expansion)
9.1.1.1. Market Revenue and Forecast (2016-2027)
9.1.2. Downstream Processing (Fill-finish, Purification)
9.1.2.1. Market Revenue and Forecast (2016-2027)
Chapter 10. Global Viral Vectors & Plasmid DNA Manufacturing Market, By End-User Type
10.1. Viral Vectors & Plasmid DNA Manufacturing Market, by End-User Type, 2020-2027
10.1.1. Biopharmaceutical and Pharmaceutical Companies
10.1.1.1. Market Revenue and Forecast (2016-2027)
10.1.2. Research Institutes
10.1.2.1. Market Revenue and Forecast (2016-2027)
Chapter 11. Global Viral Vectors & Plasmid DNA Manufacturing Market, By Disease
11.1. Viral Vectors & Plasmid DNA Manufacturing Market, by Disease, 2020-2027
11.1.1. Genetic Disorders
11.1.1.1. Market Revenue and Forecast (2016-2027)
11.1.2. Cancer
11.1.2.1. Market Revenue and Forecast (2016-2027)
11.1.3. Infectious Diseases
11.1.3.1. Market Revenue and Forecast (2016-2027)
11.1.4. Others
11.1.4.1. Market Revenue and Forecast (2016-2027)
Chapter 12. Global Viral Vectors & Plasmid DNA Manufacturing Market, Regional Estimates and Trend Forecast
12.1. North America
12.1.1. Market Revenue and Forecast, by Vector (2016-2027)
12.1.2. Market Revenue and Forecast, by Application (2016-2027)
12.1.3. Market Revenue and Forecast, by Workflow (2016-2027)
12.1.4. Market Revenue and Forecast, by End-User (2016-2027)
12.1.5. Market Revenue and Forecast, by Disease (2016-2027)
12.1.6. U.S.
12.1.6.1. Market Revenue and Forecast, by Vector (2016-2027)
12.1.6.2. Market Revenue and Forecast, by Application (2016-2027)
12.1.6.3. Market Revenue and Forecast, by Workflow (2016-2027)
12.1.6.4. Market Revenue and Forecast, by End-User (2016-2027)
12.1.7. Market Revenue and Forecast, by Disease (2016-2027)
12.1.8. Rest of North America
12.1.8.1. Market Revenue and Forecast, by Vector (2016-2027)
12.1.8.2. Market Revenue and Forecast, by Application (2016-2027)
12.1.8.3. Market Revenue and Forecast, by Workflow (2016-2027)
12.1.8.4. Market Revenue and Forecast, by End-User (2016-2027)
12.1.9. Market Revenue and Forecast, by Disease (2016-2027)
12.2. Europe
12.2.1. Market Revenue and Forecast, by Vector (2016-2027)
12.2.2. Market Revenue and Forecast, by Application (2016-2027)
12.2.3. Market Revenue and Forecast, by Workflow (2016-2027)
12.2.4. Market Revenue and Forecast, by End-User (2016-2027)
12.2.5. Market Revenue and Forecast, by Disease (2016-2027)
12.2.6. UK
12.2.6.1. Market Revenue and Forecast, by Vector (2016-2027)
12.2.6.2. Market Revenue and Forecast, by Application (2016-2027)
12.2.6.3. Market Revenue and Forecast, by Workflow (2016-2027)
12.2.7. Market Revenue and Forecast, by End-User (2016-2027)
12.2.8. Market Revenue and Forecast, by Disease (2016-2027)
12.2.8.1. Market Revenue and Forecast, by Raw Material (2016-2027)
12.2.9. Germany
12.2.9.1. Market Revenue and Forecast, by Vector (2016-2027)
12.2.9.2. Market Revenue and Forecast, by Application (2016-2027)
12.2.9.3. Market Revenue and Forecast, by Workflow (2016-2027)
12.2.10. Market Revenue and Forecast, by End-User (2016-2027)
12.2.11. Market Revenue and Forecast, by Disease (2016-2027)
12.2.12. France
12.2.12.1. Market Revenue and Forecast, by Vector (2016-2027)
12.2.12.2. Market Revenue and Forecast, by Application (2016-2027)
12.2.12.3. Market Revenue and Forecast, by Workflow (2016-2027)
12.2.12.4. Market Revenue and Forecast, by End-User (2016-2027)
12.2.13. Market Revenue and Forecast, by Disease (2016-2027)
12.2.14. Rest of Europe
12.2.14.1. Market Revenue and Forecast, by Vector (2016-2027)
12.2.14.2. Market Revenue and Forecast, by Application (2016-2027)
12.2.14.3. Market Revenue and Forecast, by Workflow (2016-2027)
12.2.14.4. Market Revenue and Forecast, by End-User (2016-2027)
12.2.15. Market Revenue and Forecast, by Disease (2016-2027)
12.3. APAC
12.3.1. Market Revenue and Forecast, by Vector (2016-2027)
12.3.2. Market Revenue and Forecast, by Application (2016-2027)
12.3.3. Market Revenue and Forecast, by Workflow (2016-2027)
12.3.4. Market Revenue and Forecast, by End-User (2016-2027)
12.3.5. Market Revenue and Forecast, by Disease (2016-2027)
12.3.6. India
12.3.6.1. Market Revenue and Forecast, by Vector (2016-2027)
12.3.6.2. Market Revenue and Forecast, by Application (2016-2027)
12.3.6.3. Market Revenue and Forecast, by Workflow (2016-2027)
12.3.6.4. Market Revenue and Forecast, by End-User (2016-2027)
12.3.7. Market Revenue and Forecast, by Disease (2016-2027)
12.3.8. China
12.3.8.1. Market Revenue and Forecast, by Vector (2016-2027)
12.3.8.2. Market Revenue and Forecast, by Application (2016-2027)
12.3.8.3. Market Revenue and Forecast, by Workflow (2016-2027)
12.3.8.4. Market Revenue and Forecast, by End-User (2016-2027)
12.3.9. Market Revenue and Forecast, by Disease (2016-2027)
12.3.10. Japan
12.3.10.1. Market Revenue and Forecast, by Vector (2016-2027)
12.3.10.2. Market Revenue and Forecast, by Application (2016-2027)
12.3.10.3. Market Revenue and Forecast, by Workflow (2016-2027)
12.3.10.4. Market Revenue and Forecast, by End-User (2016-2027)
12.3.10.5. Market Revenue and Forecast, by Disease (2016-2027)
12.3.11. Rest of APAC
12.3.11.1. Market Revenue and Forecast, by Vector (2016-2027)
12.3.11.2. Market Revenue and Forecast, by Application (2016-2027)
12.3.11.3. Market Revenue and Forecast, by Workflow (2016-2027)
12.3.11.4. Market Revenue and Forecast, by End-User (2016-2027)
12.3.11.5. Market Revenue and Forecast, by Disease (2016-2027)
12.4. MEA
12.4.1. Market Revenue and Forecast, by Vector (2016-2027)
12.4.2. Market Revenue and Forecast, by Application (2016-2027)
12.4.3. Market Revenue and Forecast, by Workflow (2016-2027)
12.4.4. Market Revenue and Forecast, by End-User (2016-2027)
12.4.5. Market Revenue and Forecast, by Disease (2016-2027)
12.4.6. GCC
12.4.6.1. Market Revenue and Forecast, by Vector (2016-2027)
12.4.6.2. Market Revenue and Forecast, by Application (2016-2027)
12.4.6.3. Market Revenue and Forecast, by Workflow (2016-2027)
12.4.6.4. Market Revenue and Forecast, by End-User (2016-2027)
12.4.7. Market Revenue and Forecast, by Disease (2016-2027)
12.4.8. North Africa
12.4.8.1. Market Revenue and Forecast, by Vector (2016-2027)
12.4.8.2. Market Revenue and Forecast, by Application (2016-2027)
12.4.8.3. Market Revenue and Forecast, by Workflow (2016-2027)
12.4.8.4. Market Revenue and Forecast, by End-User (2016-2027)
12.4.9. Market Revenue and Forecast, by Disease (2016-2027)
12.4.10. South Africa
12.4.10.1. Market Revenue and Forecast, by Vector (2016-2027)
12.4.10.2. Market Revenue and Forecast, by Application (2016-2027)
12.4.10.3. Market Revenue and Forecast, by Workflow (2016-2027)
12.4.10.4. Market Revenue and Forecast, by End-User (2016-2027)
12.4.10.5. Market Revenue and Forecast, by Disease (2016-2027)
12.4.11. Rest of MEA
12.4.11.1. Market Revenue and Forecast, by Vector (2016-2027)
12.4.11.2. Market Revenue and Forecast, by Application (2016-2027)
12.4.11.3. Market Revenue and Forecast, by Workflow (2016-2027)
12.4.11.4. Market Revenue and Forecast, by End-User (2016-2027)
12.4.11.5. Market Revenue and Forecast, by Disease (2016-2027)
12.5. Latin America
12.5.1. Market Revenue and Forecast, by Vector (2016-2027)
12.5.2. Market Revenue and Forecast, by Application (2016-2027)
12.5.3. Market Revenue and Forecast, by Workflow (2016-2027)
12.5.4. Market Revenue and Forecast, by End-User (2016-2027)
12.5.5. Market Revenue and Forecast, by Disease (2016-2027)
12.5.6. Brazil
12.5.6.1. Market Revenue and Forecast, by Vector (2016-2027)
12.5.6.2. Market Revenue and Forecast, by Application (2016-2027)
12.5.6.3. Market Revenue and Forecast, by Workflow (2016-2027)
12.5.6.4. Market Revenue and Forecast, by End-User (2016-2027)
12.5.7. Market Revenue and Forecast, by Disease (2016-2027)
12.5.8. Rest of LATAM
12.5.8.1. Market Revenue and Forecast, by Vector (2016-2027)
12.5.8.2. Market Revenue and Forecast, by Application (2016-2027)
12.5.8.3. Market Revenue and Forecast, by Workflow (2016-2027)
12.5.8.4. Market Revenue and Forecast, by End-User (2016-2027)
12.5.8.5. Market Revenue and Forecast, by Disease (2016-2027)
Chapter 13. Company Profiles
13.1. Novasep
13.1.1. Company Overview
13.1.2. Product Offerings
13.1.3. Financial Performance
13.1.4. Recent Initiatives
13.2. Aldevron
13.2.1. Company Overview
13.2.2. Product Offerings
13.2.3. Financial Performance
13.2.4. Recent Initiatives
13.3. Merck Waisman Biomanufacturing
13.3.1. Company Overview
13.3.2. Product Offerings
13.3.3. Financial Performance
13.3.4. Recent Initiatives
13.4. Creative Biogene
13.4.1. Company Overview
13.4.2. Product Offerings
13.4.3. Financial Performance
13.4.4. Recent Initiatives
13.5. The Cell and Gene Therapy Catapult
13.5.1. Company Overview
13.5.2. Product Offerings
13.5.3. Financial Performance
13.5.4. Recent Initiatives
13.6. Cobra Biologics
13.6.1. Company Overview
13.6.2. Product Offerings
13.6.3. Financial Performance
13.6.4. Recent Initiatives
13.7. uniQure N.V.
13.7.1. Company Overview
13.7.2. Product Offerings
13.7.3. Financial Performance
13.7.4. Recent Initiatives
13.8. Addgene
13.8.1. Company Overview
13.8.2. Product Offerings
13.8.3. Financial Performance
13.8.4. Recent Initiatives
13.9. FUJIFILM Holdings Corporation
13.9.1. Company Overview
13.9.2. Product Offerings
13.9.3. Financial Performance
13.9.4. Recent Initiatives
13.10. Oxford Biomedicaplc
13.10.1. Company Overview
13.10.2. Product Offerings
13.10.3. Financial Performance
13.10.4. Recent Initiatives
13.11. Takara Bio Inc.
13.11.1. Company Overview
13.11.2. Product Offerings
13.11.3. Financial Performance
13.11.4. Recent Initiatives
Chapter 14. Research Methodology
14.1. Primary Research
14.2. Secondary Research
14.3. Assumptions
Chapter 15. Appendix
15.1. About Us
15.2. Glossary of Terms
Buy this Premium Research Report@ https://www.precedenceresearch.com/checkout/1012
About Us:
Precedence Research is a worldwide market research and consulting organization. We give unmatched nature of offering to our customers present all around the globe across industry verticals. Precedence Research has expertise in giving deep-dive market insight along with market intelligence to our customers spread crosswise over various undertakings. We are obliged to serve our different client base present over the enterprises of medicinal services, healthcare, innovation, next-gen technologies, semi-conductors, chemicals, automotive, and among different ventures present globally.
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com



0 Comments