Precedence Research, Recently Published Report on “Anesthesia and Respiratory Devices Market Size, Share, Growth, Trends, Segment Forecasts, Regional Outlook 2020 - 2027”. The report offers an up-to-date analysis regarding the current market scenario, latest trends, key drivers, potential challenges, profitability graph and the overall market environment.
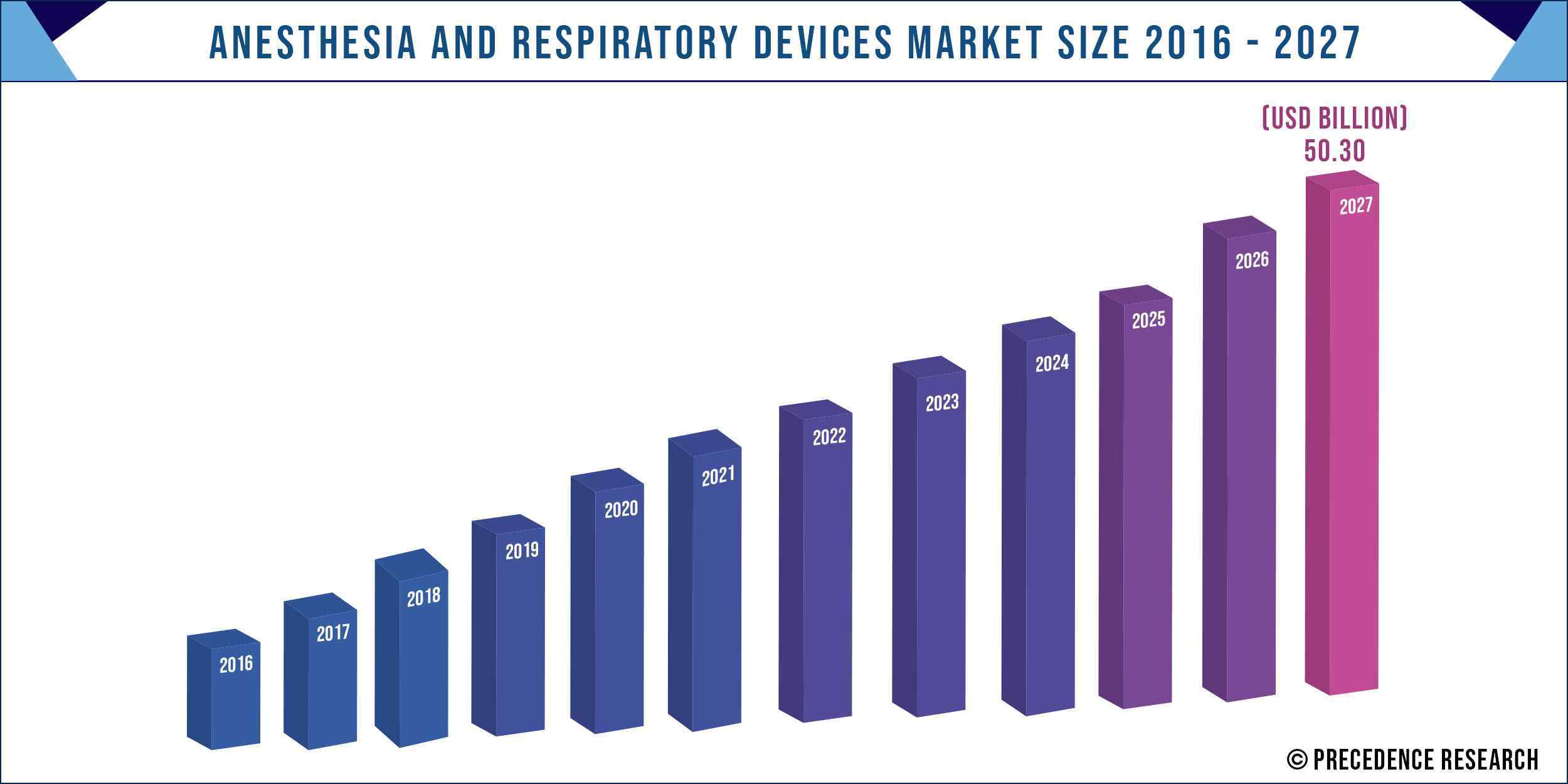
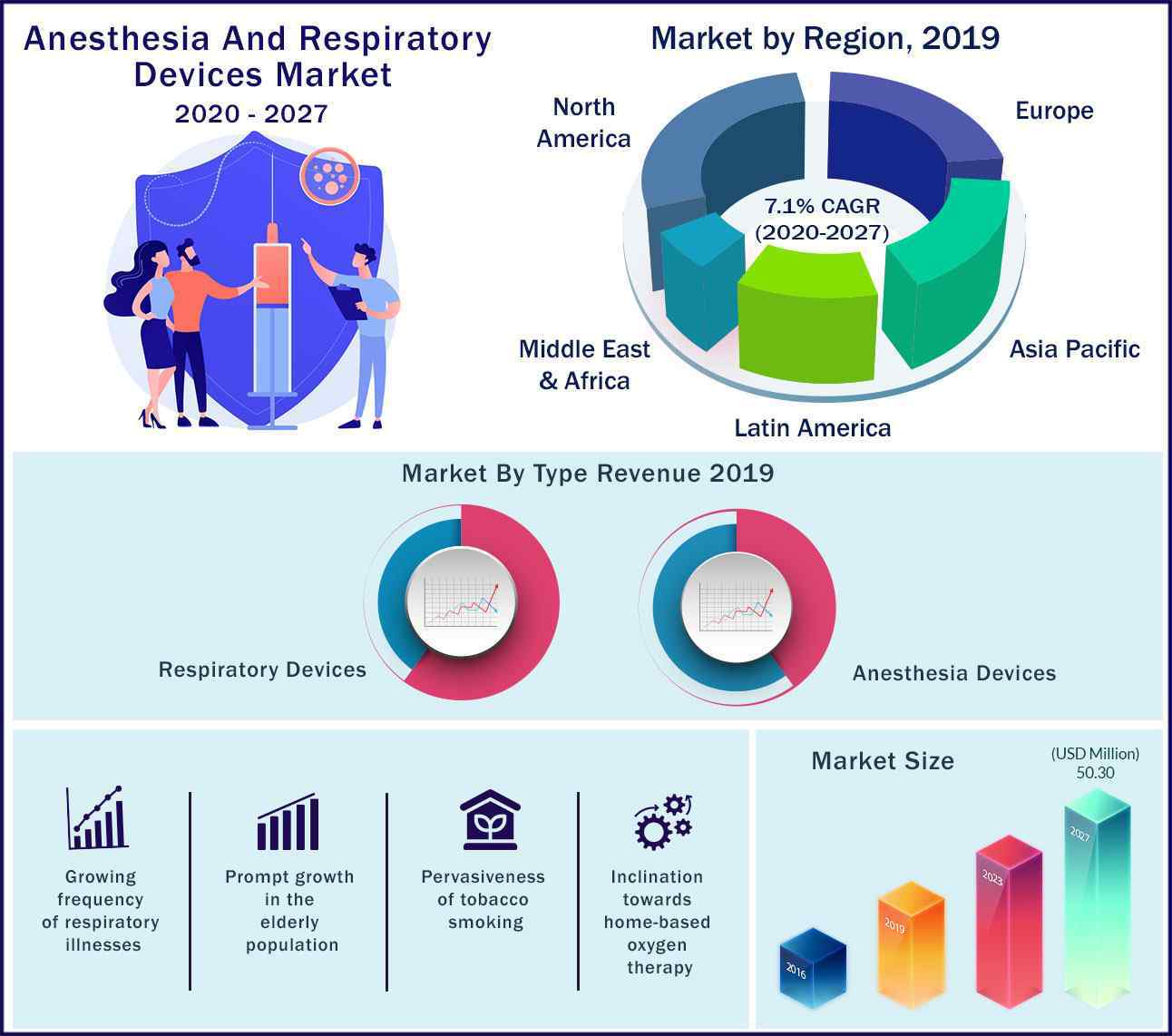
The global Anesthesia and Respiratory Devices Market value is projected to reach US Dollar 50.3 billion by 2027 and is projected to expand at a CAGR of ~7.01% from 2020 to 2027.
An important feature when governing superior respiratory devices is the prompt detection of the patient's breath phase with the help of a flow trigger. With help of this method device can support a spontaneous breath with a preset over pressure, whereas maintaining the patient's respiratory work to a smallest. At the same time, the dimensions requisite to be extremely accurate over the entire flow range for numerous treatments to perceive the patient's respiratory pattern reliably.
Nowadays, anesthesia machines are being progressively combined in healthcare systems as they are seen as an important medical apparatus. The aim of anesthesia care is to safeguard the safest promising practice for the patient. The arrival of catheter systems, improved needles, ultrasound, and observing has completely revived, if not transformed, the practice of anesthesia. Anesthesia machines have progressed from simple, pneumatic devices to refined, computer-based, and fully incorporated systems. The progressive versions of the machines provide greater patient convenience and protection.
Get the Sample Pages of Report for More Understanding@ https://www.precedenceresearch.com/sample/1098
Growth Factors
Progresses throughout the past several decades have led to imperative improvements in clinical-practice development and clinical-monitoring technology. Not only in patients being cared for in intensive care units or patients undertaking surgery but also in ambulatory patients. Additionally, anesthesiologists across the globe have established standards for uninterrupted real-time observing of oxygenation, hemodynamics, neurological status, ventilation, degree of neuromuscular blockade, urine output, core temperature and other that have also backed suggestively to safety of patient.
Further, rising cases of respiratory diseases, speedy growth in the amount of surgical procedures, prompt urbanization, mounting population are some of the major factors driving the market growth. Further, escalating elderly population and upsurge in tobacco consumption are further pushing the demand for anesthesia and respiratory devices worldwide. Furthermore, modernizations in the field of respiratory care devices and prolonged levels of governmental upkeep in approving these devices complement the market landscape. Promptly developing demand for therapeutic devices in homecare settings and noteworthy upsurge in the healthcare expenses are other aspects expected to bid profitable growth prospects for the market during years to come.
Report Highlights
- Presently, anesthesia equipment industry is trending near a compacted ergonomic design for simplicity of use and surfaces that are simple to keep clean to decrease nosocomial infections. This apparatus has integrated revolutionary monitoring that is multipurpose and customizable to upturn diagnostic assurance.
- Asia Pacific is projected to eyewitness the firmest growth over the estimate period due to cumulative healthcare spending and responsiveness among patients in this region
- In 2019, North America dominated the global anesthesia & respiratory devices market due to cumulative adoption of technologically innovative devices, growing investments by the pharmaceutical firms, and intensifying healthcare spending.
Buy this Premium Research Report, Ask Here@ https://www.precedenceresearch.com/checkout/1098
Regional Snapshots
The anesthesia and respiratory devices market in the U.S. would propagate, as the number of people suffering from COPD is mounting speedily, and it is assessed that nearby 12 million patients are suffering from COPD in the U.S. Growing aging population, escalating number of outpatient operations accomplished and obtainability of compensation for respiratory and anesthesia devices are some of the prime reasons that are motivating the growth of the anesthesia and respiratory devices market in the U.S.
Key Players & Strategies
Vital players are taking initiatives to improve innovative instruments with an intention to convey enhancement in the treatment. For illustration, B. Braun Medical Inc. introduced Clorotekal, a U.S. Food and Drug Administration (FDA) permitted anesthetics for spinal anesthesia in April 2018. Such initiatives from major player are projected to push growth of the anesthesia and respiratory devices market in the upcoming years.
Some of the prominent company’s operative in this market includes Philips Healthcare, Medtronic Plc., Getinge Group, Masimo, Draegerwerk AG, Smith's Medical, ResMed and Teleflex Inc., OSI Systems among others. Other noticeable companies in the value chain are Verathon Inc., Aircraft Medical, Karl Storz GmbH & Co. KG, Medline Industries Inc., Pentax, 3B Medical, Inc., Acare Technology Co., Ltd., Hill-Rom Holdings Inc., Allied Healthcare Inc., and Rotech Healthcare Inc.
Segments Covered in the Report
This research report includes complete assessment of the market with the help of extensive qualitative and quantitative insights, and projections regarding the market. This report offers breakdown of market into prospective and niche sectors. Further, this research study calculates market revenue and its growth trend at global, regional, and country from 2016 to 2027. This report includes market segmentation and its revenue estimation by classifying it on the basis of product and region as follows:
By Product
- Anesthesia Devices
- Machines
- Workstations
- Monitors
- Ventilators
- Delivery machines
- Standalone
- Portable
- Disposables
- Disposable Accessories
- Disposable Masks
- Respiratory Devices
- Disposables
- Disposable Oxygen Masks
- Resuscitators
- Tracheostomy Tubes
- Oxygen Cannula
- Equipment
- Reusable Resuscitators
- Ventilators
- Adult Ventilators
- Neonatal ventilators
- Positive Airway Pressure
- Bi-level positive airway pressure devices
- Continuous positive airway pressure devices
- Nebulizers
- Pneumatic nebulizers
- Mesh nebulizers
- Ultrasonic nebulizers
- Inhalers
- Dry powdered inhalers
- Metered-dose inhaler
- Humidifiers
- Heat exchangers
- Pass over humidifiers
- Heat humidifiers
- Heated wire breathing circuits
- Oxygen Concentrators
- Portable oxygen concentrators
- Fixed oxygen concentrators
- Measurement Devices
- Spirometers
- Pulse Oximeters
- Capnography
- Peak Flow Meters
- Disposables
- Machines
By Regional Outlook
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Asia Pacific
- China
- India
- Japan
- South Korea
- Middle East & Africa
- Latin America
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. Market Dynamics Analysis and Trends
5.1. Market Dynamics
5.1.1. Market Drivers
5.1.2. Market Restraints
5.1.3. Market Opportunities
5.2. Porter’s Five Forces Analysis
5.2.1. Bargaining power of suppliers
5.2.2. Bargaining power of buyers
5.2.3. Threat of substitute
5.2.4. Threat of new entrants
5.2.5. Degree of competition
Chapter 6. Competitive Landscape
6.1.1. Company Market Share/Positioning Analysis
6.1.2. Key Strategies Adopted by Players
6.1.3. Vendor Landscape
6.1.3.1. List of Suppliers
6.1.3.2. List of Buyers
Chapter 7. Global Anesthesia and Respiratory Devices Market, By Product
7.1. Anesthesia and Respiratory Devices Market, by Product Type, 2020-2027
7.1.1. Anesthesia Devices
7.1.1.1. Machines
7.1.1.2. Disposables
7.1.1.3. Market Revenue and Forecast (2016-2027)
7.1.2. Respiratory Devices
7.1.2.1. Disposables
7.1.2.2. Equipment
7.1.2.3. Oxygen Concentrators
7.1.2.4. Market Revenue and Forecast (2016-2027)
7.1.3. Measurement Devices
7.1.3.1. Spirometers
7.1.3.2. Pulse Oximeters
7.1.3.3. Capnography
7.1.3.4. Peak Flow Meters
7.1.3.5. Market Revenue and Forecast (2016-2027)
Chapter 8. Global Anesthesia and Respiratory Devices Market, Regional Estimates and Trend Forecast
8.1. North America
8.1.1. Market Revenue and Forecast, by Product (2016-2027)
8.1.2. U.S.
8.1.3. Rest of North America
8.1.3.1. Market Revenue and Forecast, by Product (2016-2027)
8.2. Europe
8.2.1. Market Revenue and Forecast, by Product (2016-2027)
8.2.2. UK
8.2.2.1. Market Revenue and Forecast, by Product (2016-2027)
8.2.3. France
8.2.3.1. Market Revenue and Forecast, by Product (2016-2027)
8.2.4. Rest of Europe
8.2.4.1. Market Revenue and Forecast, by Product (2016-2027)
8.3. APAC
8.3.1. Market Revenue and Forecast, by Product (2016-2027)
8.3.2. India
8.3.2.1. Market Revenue and Forecast, by Product (2016-2027)
8.3.3. China
8.3.3.1. Market Revenue and Forecast, by Product (2016-2027)
8.3.4. Japan
8.3.4.1. Market Revenue and Forecast, by Product (2016-2027)
8.3.5. Rest of APAC
8.3.5.1. Market Revenue and Forecast, by Product (2016-2027)
8.4. MEA
8.4.1. Market Revenue and Forecast, by Product (2016-2027)
8.4.2. GCC
8.4.2.1. Market Revenue and Forecast, by Product (2016-2027)
8.4.3. North Africa
8.4.3.1. Market Revenue and Forecast, by Product (2016-2027)
8.4.4. South Africa
8.4.4.1. Market Revenue and Forecast, by Product (2016-2027)
8.4.5. Rest of MEA
8.4.5.1. Market Revenue and Forecast, by Product (2016-2027)
8.5. Latin America
8.5.1. Market Revenue and Forecast, by Product (2016-2027)
8.5.2. Brazil
8.5.2.1. Market Revenue and Forecast, by Product (2016-2027)
8.5.3. Rest of LATAM
8.5.3.1. Market Revenue and Forecast, by Product (2016-2027)
Chapter 9. Company Profiles
9.1. Masimo Corporation
9.1.1. Company Overview
9.1.2. Product Offerings
9.1.3. Financial Performance
9.1.4. Recent Initiatives
9.2. Medtronic
9.2.1. Company Overview
9.2.2. Product Offerings
9.2.3. Financial Performance
9.2.4. Recent Initiatives
9.3. AirSep Corporation
9.3.1. Company Overview
9.3.2. Product Offerings
9.3.3. Financial Performance
9.3.4. Recent Initiatives
9.4. Smiths Medical
9.4.1. Company Overview
9.4.2. Product Offerings
9.4.3. Financial Performance
9.4.4. Recent Initiatives
9.5. GE Healthcare
9.5.1. Company Overview
9.5.2. Product Offerings
9.5.3. Financial Performance
9.5.4. Recent Initiatives
9.6. Philips Healthcare
9.6.1. Company Overview
9.6.2. Product Offerings
9.6.3. Financial Performance
9.6.4. Recent Initiatives
9.7. B. Braun Medical Inc.
9.7.1. Company Overview
9.7.2. Product Offerings
9.7.3. Financial Performance
9.7.4. Recent Initiatives
9.8. Drägerwerk AG & Co
9.8.1. Company Overview
9.8.2. Product Offerings
9.8.3. Financial Performance
9.8.4. Recent Initiatives
9.9. KGaA
9.9.1. Company Overview
9.9.2. Product Offerings
9.9.3. Financial Performance
9.9.4. Recent Initiatives
9.10. Getinge AB
9.10.1. Company Overview
9.10.2. Product Offerings
9.10.3. Financial Performance
9.10.4. Recent Initiatives
9.11. Teleflex Inc
9.11.1. Company Overview
9.11.2. Product Offerings
9.11.3. Financial Performance
9.11.4. Recent Initiatives
Chapter 10. Research Methodology
10.1. Primary Research
10.2. Secondary Research
10.3. Assumptions
Chapter 11. Appendix
11.1. About Us
11.2. Glossary of Terms
Buy this Premium Research Report@ https://www.precedenceresearch.com/checkout/1098
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 774 402 6168
Contact Us:
Precedence Research
Apt 1408 1785 Riverside Drive Ottawa, ON, K1G 3T7, Canada
Call: +1 774 402 6168
Email: sales@precedenceresearch.com



0 Comments